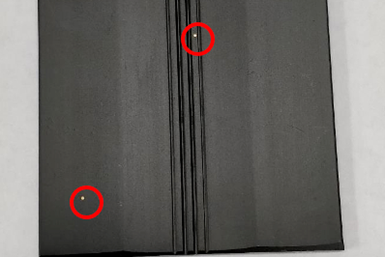
Anodizing is a process used to create an aluminum oxide film on the surface of aluminum or aluminum alloy products. It involves placing the aluminum or aluminum alloy product as the anode in an electrolyte solution and applying an electric current to form the aluminum oxide film. Anodizing improves the corrosion resistance, wear resistance, and decorative properties of aluminum profiles. During the anodizing process of aluminum profiles, several common defect features can occur. Let’s primarily understand the causes of spotted defects. Material corrosion, bath contamination, precipitation of alloy second phases, or galvanic effects can all lead to spotted defects. They are described as follows:
1.Acid or alkali etching
Before anodizing, the aluminum material may be corroded by acid or alkaline liquids, or affected by acid or alkaline fumes, resulting in localized white spots on the surface. If the corrosion is severe, larger pitting spots can form. It is difficult to determine with the naked eye whether the corrosion is caused by acid or alkali, but it can be easily distinguished by observing the cross-section of the corroded area under a microscope. If the bottom of the pit is round and without intergranular corrosion, it is caused by alkali etching. If the bottom is irregular and accompanied by intergranular corrosion, with deeper pits, it is caused by acid etching. Improper storage and handling in the factory can also lead to this type of corrosion. Acid fumes from chemical polishing agents or other acidic fumes, as well as chlorinated organic degreasers, are sources of acid etching. Common alkali etching is caused by the scattering and splashing of mortar, cement ash, and alkaline washing liquids. Once the cause is determined, strengthening the management of various processes in the factory can solve the problem.
2.Atmospheric corrosion
Aluminum profiles exposed to humid air may develop white spots, which often align longitudinally along the mold lines. Atmospheric corrosion is generally not as severe as acid or alkali etching and can be removed by mechanical methods or alkaline washing. Atmospheric corrosion is mostly non-localized and tends to occur on certain surfaces, such as lower temperature areas where water vapor easily condenses or on upper surfaces. When atmospheric corrosion is more severe, the cross-section of the pitting spots appears like inverted mushrooms. In this case, alkaline washing cannot eliminate the pitting spots and may even enlarge them. If atmospheric corrosion is determined, the storage conditions in the factory should be checked. Aluminum materials should not be stored in areas with excessively low temperatures to prevent water vapor condensation. The storage area should be dry, and the temperature should be as uniform as possible.
3.Paper corrosion (water spots)
When paper or cardboard is placed between aluminum materials or used for packaging, it prevents abrasion. However, if the paper becomes damp, corrosion spots appear on the surface of the aluminum. When corrugated cardboard is used, regular lines of corrosion spots appear at the points of contact with the corrugated board. Although defects may sometimes be visible directly on the aluminum surface, they are often more pronounced after alkaline washing and anodizing. These spots are generally deep and difficult to remove by mechanical means or alkaline washing. Paper (board) corrosion is caused by acid ions, mainly SO42- and Cl-, which are present in the paper. Therefore, using paper (board) without chlorides and sulfates and avoiding water penetration are effective methods to prevent paper (board) corrosion.
4.Cleaning water corrosion (also known as snowflake corrosion)
After alkaline washing, chemical polishing, or sulfuric acid pickling, if the rinsing water contains impurities, it may result in star-shaped or radiating spots on the surface. The corrosion depth is shallow. This type of corrosion occurs when the cleaning water is heavily contaminated or when the flow rate of overflow rinsing is low. It resembles snowflake-shaped crystals in appearance, hence the name “snowflake corrosion.” The cause is the reaction between impurities of zinc in the aluminum and the SO42- and Cl- in the cleaning water. If the insulation of the tank is poor, galvanic effects can exacerbate this defect. According to foreign sources, when the content of Zn in the aluminum alloy is greater than 0.015%, the Cl- in the cleaning water is higher than 15 ppm, this type of corrosion is likely to occur. Using nitric acid for pickling or adding 0.1% HNO3 to the cleaning water can eliminate it.
5.Chloride corrosion
The presence of a small amount of chloride in the sulfuric acid anodizing bath can also lead to pitting corrosion. The characteristic appearance is deep black star-shaped pits, which are more concentrated at the edges and corners of the workpiece or in other areas with higher current densities. The pitting locations do not have an anodized film, and the thickness of the film in the remaining “normal” areas is lower than the expected value. The high salt content in tap water is the main source of Cl- pollution in the bath.
6.Galvanic corrosion
In an energized tank (anodizing or electrolytic coloring), the galvanic effects between the workpiece and the tank (steel tank), or the effects of stray currents in a non-energized tank (rinsing or sealing), can cause or aggravate pitting corrosion.
Edited by May Jiang from MAT Aluminum
Post time: Dec-15-2023